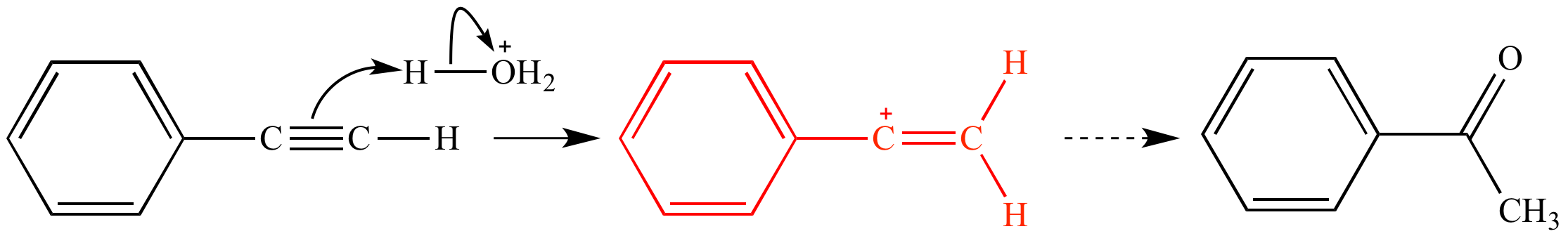
In the first mechanism step the alkyne is protonated by hydronium ion a strong acid to produce a resonance stabilized secondary vinylic carbocation shown in red.
Vinylic carbocation definition.
The vinyl cation is a carbocation with the positive charge on an alkene carbon.
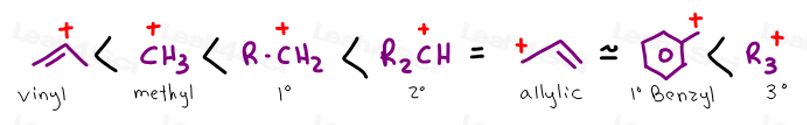
An allylic carbocation in which an allylic carbon bears the positive charge.
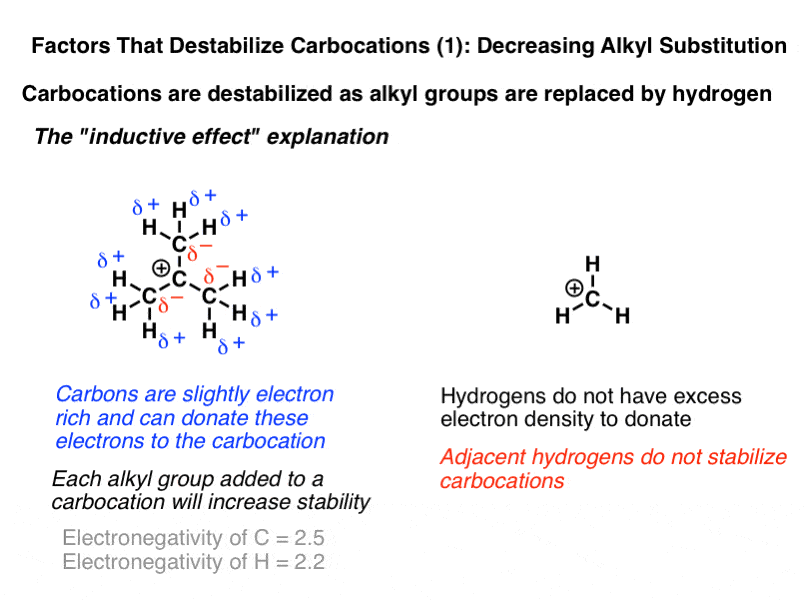
For a positive center to be attached to a more electronegative group is destabilizing.
Its empirical formula is c 2h 3.
Formerly it was known as carbonium ion.
A vinylic carbocation which has an empirical formula of c h is a carbocation that has a positive charge only on the alkene carbon atom.
Carbocation today is defined as any even electron cation that possesses a significant positive charge on the carbon atom.
This carbocation is also a benzylic carbocation.
The univalent hydrocarbon group ch 2 ch derived from ethylene.
We can basically say that they are carbon cations.
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds.
Atoms or groups attached to an allylic carbon are termed allylic substituents.
Look at the figure below notice that in the alkyl carbocation on the left the cationic center is attached to an s p x 3 carbon whereas in the vinylic cation in the middle the cationic center is attached to a more electronegative s p x 2 carbon.
A carbocation with a two coordinate positive carbon derived from formal removal of a hydride ion h from an alkene is known as a vinyl cation.
An allylic carbon is an sp3 carbon that is adjacent to a vinylic carbon.
Any trivalent disubstituted carbon is generally a vinylic carbocation in which the carbon atom which is bearing the positive charge is found to be double bonded and will always exist as sp hybridized.